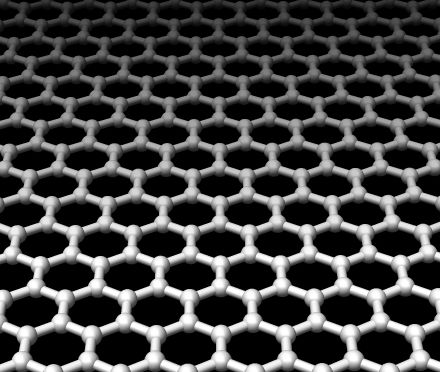
 The researchers arranged flakes of graphene over holes in a silicon wafer and pressed down on them with the diamond tip of an atomic force microscope to determine how much force the graphene flakes could withstand before rupturing. They found that they could push down about 100 nanometers with a force of up to 2.9 micronewtons before the graphene flakes would rupture.
The researchers arranged flakes of graphene over holes in a silicon wafer and pressed down on them with the diamond tip of an atomic force microscope to determine how much force the graphene flakes could withstand before rupturing. They found that they could push down about 100 nanometers with a force of up to 2.9 micronewtons before the graphene flakes would rupture.That may not sound like much, but if engineers were able to produce a sheet of graphene as thick as ordinary plastic wrap that you use to cover your food dishes, it would take the weight of a heavy car to tear through it. Of course, that's an unrealistic analogy, as graphene is—by definition—only a single atomic layer in thickness, and therefore can't be as thick as a sheet of plastic wrap. But on the nano-scale, graphene has tremendous strength, and it could be added to polymers to form super-strength composites.
Besides its superior strength, graphene has a number of other interesting properties: it has unusually high opacity for an atomic monolayer, it is a zero-gap semiconductor, has remarkably high electron mobility at room temperature, displays an anomalous quantum hall effect in the presence of a magnetic field, and has unexpectedly high thermal conductivity.


No comments:
Post a Comment